

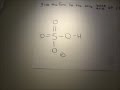















In this example, sulfuric acid (H 2SO4) is an acid because it "donates" H + to the water. It becomes the hydrogen sulfite ion (H SO− 4) which is the conjugate base of sulfuric acid. The same idea applies to a base: N H 3 + H 2O <=> N H + 4 + OH − what is the conjugate acid for ClO4^-what is the conjugate base for H2SO4. what is the conjugate acid for HSO4^- What is the conjugate base of HSO4−? Express your answer as a chemical formula. What is the conjugate acid of HPO42− ? Express your answer as a chemical formula. Videos. Step-by-step answer 100% (2 rating) 01:43 2 0. Expert Answer 98% (66 ratings) Previous question Next question ... Click here👆to get an answer to your question ️ The conjugate base of HSO4^- in an aqueous solution is: While in theory HSO4- the conjugate base of sulfuric acid, in aqueous solution it will never actually be a base, because it won't accept a proton to make molecules of H2SO4. As I explained, H2SO4... Which is a conjugate acid-base pair in the following equation? H2SO4 + H2O ----> HSO4 -1 + H3O +1 a H2SO4 and H2O b H3O +1 and H2SO4 c HSO4 -1 and H3O +1 d H2SO4 and HSO4 -1 Here's what I got. The thing to remember about conjugate acids is that they are the chemical species that is formed when a Bronsted-Lowry base accepts one proton, "H"^(+). This means that in order to find the conjugate acid of a substance that can act as a Bronsted-Lowry base, all you have to do is add a proton to it. But keep in mind that a proton carries a 1+ charge, so make sure that you ... Problem: Identify the conjugate acid of SO42–.a. H2SO4b. HSO4–c. H2SO3d. H3O+e. SO32– FREE Expert Solution Show answer. 80% (240 ratings) Problem Details. Identify the conjugate acid of SO 4 2–. a. H 2 SO 4 b. HSO 4 – c. H 2 SO 3 d. H 3 O + e. SO 3 2– Learn this topic by watching Conjugate Acids and Bases Concept Videos. All Chemistry Practice Problems Conjugate Acids and Bases ... In each of the following chemical equations, identify the conjugate acid-base pairs. a. HSO4- + H2O ( SO42- + H3O+ b. CH3NH2 + HCl ( CH3NH3+ + Cl-c. CH3COO- + H2O ( CH3COOH + OH-Complete the equation for each of the following conjugate acid-conjugate base reactions. HNO2 + H2O ( H3O+ + NO2- What is the conjugate base of hso4? HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. The conjugate base of HSO4- is SO4 (2-), if that’s the question that you are asking. Is CN an acid or base?
[index] [455] [2081] [6878] [1716] [4739] [8565] [1209] [5966] [522] [7864]
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Draw CH3NO2 (Nitromethane) in Lewis and its conjugate acid - Duration: 61 seconds. 11,751 views; 4 years ago; 1:04. Give the formula of the conjugate acid/base of HSO4^(-) - Duration: 64 seconds ... Identify the conjugate acid-conjugate base pairs in the following chemical equations:HCOOH (aq) + CN- (aq) = HCOO- (aq) + HCN (aq)H2SO4 (aq) + N2H5+ (aq) = H... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com 068 - Acid-Base EquilibriumIn this video Paul Andersen explains how acid-base chemistry can be understood in terms of equilibrium. Water is present in all a... Acid Base Chemistry 4c. ... or 4 electronegative oxygen atoms present, as in the case of the HSO4- ion, ... 7a. pKa and pKb of conjugate acids and bases - Duration: 6:46. Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... Covers the HSC chemistry syllabus dot point: "identify amphiprotic substances and construct equations to describe their behaviour in acidic and basic solutions"
Copyright © 2024 m.hotkazino.space